Abstract
Background Autologous stem cell transplant (ASCT) remains a mainstay of therapy in Multiple Myeloma (MM) management. At the time of stem cell collection, it is recommended to collect stem cells for two transplants. However, the use of stem cells for a tandem and salvage auto transplant has decreased over time. Here, we evaluate the trends in stem cell collection and utilization over the period of 2014 to mid 2020 at a large urban medical center.
Methods We identified 155 patients with MM in the EPIC database at Montefiore Medical Center who had their stem cells collected with the purpose of autologous stem cell transplant from January 2014 to July 2020. We extracted data on patient characteristics (age, sex, race, and ethnicity), treatment factors (time from diagnosis to stem cell mobilization and induction regiments), as well as MM disease characteristics (heavy and light chain subtypes) and compared them to stem cell utilization, the number of ASCTs received, to identify trends.
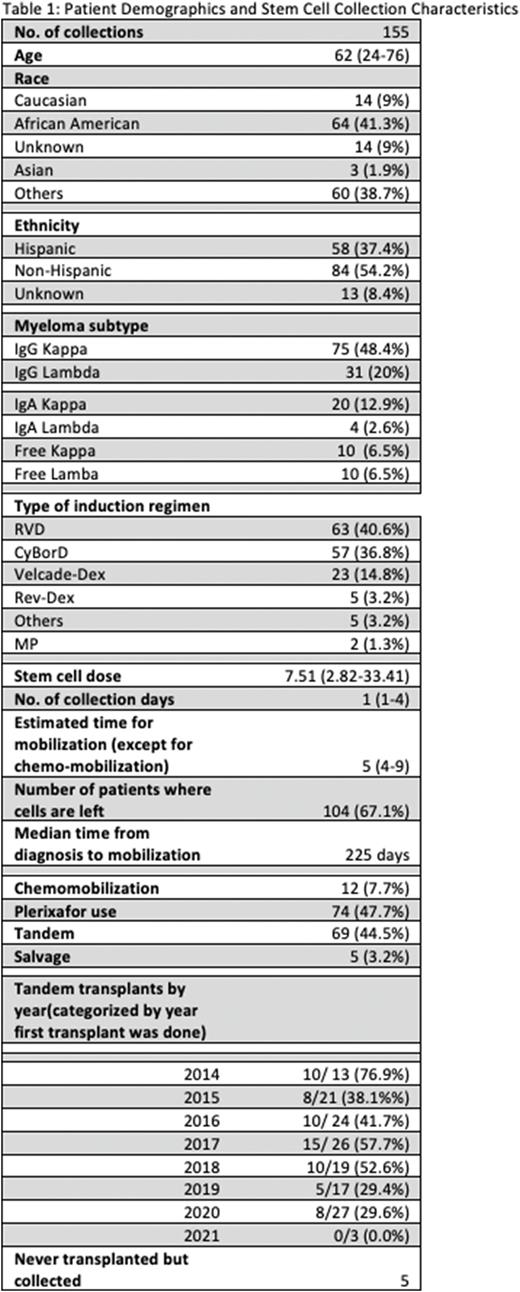
Results Patient demographics and information on disease characteristics are shown in Table 1. From January 2014 to July 2020, 155 patients underwent at least one apheresis session. The median number of apheresis sessions was 1 (Range 1-4). The median number of CD34 stem cells collected was 7.51 x10^6 CD34 cells/kg (2.82-33.41). The median time from MM diagnosis to apheresis was 225 days (range 82-1713). Chemo-mobilization was done in 12 patients (7.7%). Plerixafor was used in 74 patients (47.7%). Excluding those requiring chemo-mobilization, it took median of 5 days from the initiation of mobilization to collection (range 4-9). Of the 155 patients, 150 have undergone a transplant. To this date, 69 (44.5%) had a tandem transplant and only 5 patients (3.2%) had a salvage auto transplant. 104 patients (67.1%) had stem cells left.
The median number of infused CD34 cells was 3.84 x10^6 CD34 cells/ kg (2.4-16.4) for the first transplant and 3.7 x 10^6 CD34 cells/ kg (2.3-9.77) for the second transplant. Table 1 shows the number of patients undergoing tandem transplant over the years.
The median overall survival (OS) for the entire cohort was 34.4 months (156-2909 days). 131 patients (84.5%) are alive at the time of last follow-up.
Conclusion Our study highlights the stem cell collection and utilization trends for Multiple Myeloma at a tertiary care hospital that is responsible for care of minority ethnic groups. Our data shows significant utilization of tandem transplant, however, the use is decreasing over the years. More than half of our patients still have cryopreserved stem cells left and will possibly not be used for a second transplant. It may be time to revise our apheresis goal for myeloma patients since a significant portion of patients may not end up going to a second autologous stem cell transplant. However, the utilization of stem cells in other settings is also evolving. Although tandem and salvage transplant have fallen out of favor, it is possible that with the advent of newer therapies such as CAR-T cells and bispecific T-cell engagers, these stem cells may be used in alternative setting post treatment for prolonged cytopenia. Our next steps would be to highlight the outcomes of patients undergoing tandem transplant at our center as compared to those undergoing one autologous stem cell transplant as well as report on the trends of stem cell use post novel-therapies such CAR-T cell therapy.
Disclosures
Shastri:Janssen: Consultancy; Rigel Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; NACE: Honoraria; Kymera Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Shah:Janssen: Consultancy, Research Funding; MJH Lifesciences: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Research Funding; ACCC: Honoraria; MashUpMD: Honoraria; Sanofi: Consultancy. Verma:Eli Lilly and Company: Research Funding; Celgene: Consultancy, Research Funding; MedPacto: Research Funding; Stelexis Therapeutics: Consultancy; Stelexis Therapeutics: Current equity holder in publicly-traded company; Curis: Research Funding; Novartis: Consultancy, Research Funding; Incyte: Research Funding; Jannsen: Research Funding; BMS: Research Funding; Throws Exception: Current equity holder in publicly-traded company; Acceleron Pharma: Consultancy; GlaxoSmithKline: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.